
Dr. Ray Franco, Ph.D., PE, is an electrical and electronics engineer. He is a Forensic Smoke Alarm Expert. He is a "Certified Fire Alarm ITM Specialist" by the National Fire Protection Association (NFPA) - Certification No.: CFAITMS-FM-18-047. His Curriculum Vitae (CV) is in the menu at the bottom of this page. His phone number and email address are in the header of this page. Dr. Franco does both plaintiff and defense work. Your first consulting phone call is free.
Smoke Alarms are self-contained, autonomous units that include the detection and warning components in one unit. Smoke alarms may be single station or multiple station. Multiple-station alarms can be interconnected so that if one alarms, they all alarm. Smoke alarms do not require a control unit for supervision or power. They are primarily used in household dwelling units.
The horn in a smoke alarm produces a 3,100 Hz tone at a sound level of 85 dB-A. The sound-pressure waves will cause enhanced soot depositions on the horn grill and openings. This can be used by forensic investigators of smoke alarms to determine whether the smoke alarm sounded.

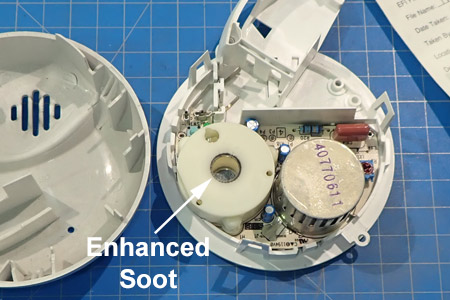
In addition to there being enhanced soot depositions around the horn opening, there are soot depositions around the horn hole on the inside. Dr. Franco has done some pioneering work in this area - Acoustic Soot.
The first step in any smoke alarm litigation is identifying the manufacturer, then the model number and age. This can also be one of the most difficult steps. There was a fire. The plastic housing may have been consumed in the fire, or even worst what is left of the smoke alarm is in melted and re-solidified plastic. Code law and manufactures state to replace smoke alarms every 10 years. The dilemma is, if the smoke alarm is 20 years old, you are probably not going to have a good legal case against the manufacturer, and if it is less than 10 years old, you probably do not want to file a lawsuit against the landlord. Identification of smoke alarms is an area where Dr. Franco excels.
In the case presented below, the smoke alarm was partially encapsulated in re-solidified plastic. Dr. Franco was able to identify the manufacture from an x-ray that depicted the piezoelectric disc in the horn and the small printed circuit board behind it.
Once the manufacture was identified, Dr. Franco was able to determine the model by matching the back of the smoke alarm printed circuit board to one of his exemplars.
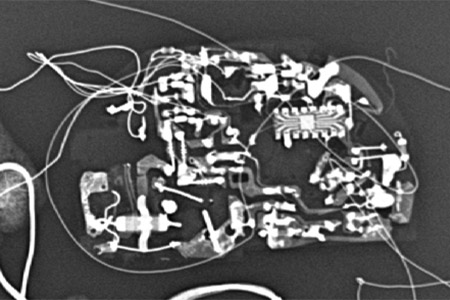
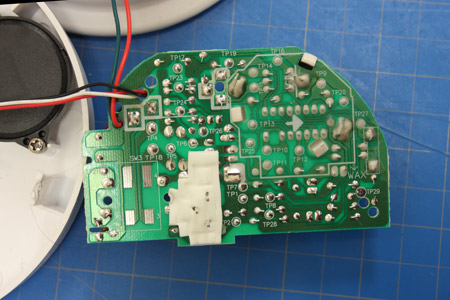
This model was still in production so there was not a lot of emphasis on determining its age.
Often printed circuit boards have revision numbers etched in copper on them, and they will survive. If you have enough exemplars, you can narrow the age down pretty close. For an example, click here
Another way of determining the age is via the Underwriters Laboratories (UL) issue number. Dr. Franco keeps a database of UL issue numbers for all of the smoke alarms he has examined. For an exemple, click here
Smoke alarm product defects are extremely rare, but they do happen. In this case, the smoke alarm failed to wake the occupant. All of the other experts were so busy looking for enhanced soot depositions around the horn-opening that they missed the defect. Dr. Franco found the defect: spring Pin #1 is on the wrong side of the the piezoelectric horn-disc.
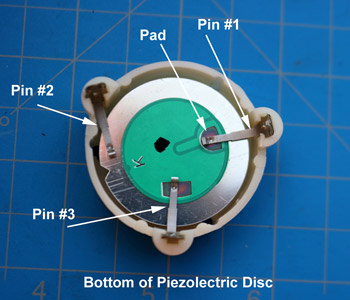
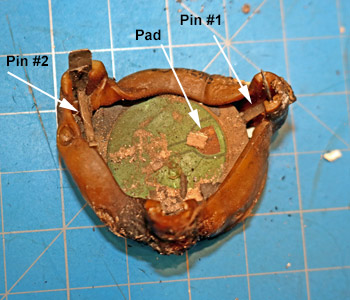
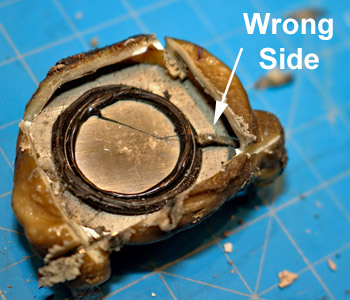
Although product defects are rare, there have been a number of smoke alarm recalls. In 2018, Kidde recalled 500,000 smoke alarms because they were assembled without removing the protective cap on one of the sensors - CPSC Recall No.: 18-128.